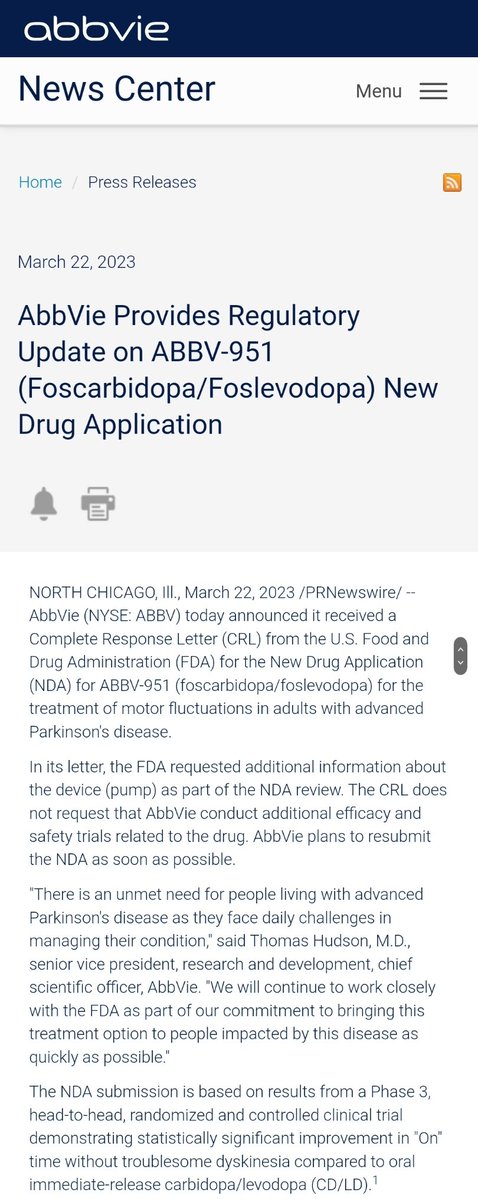
AbbVie Provides Regulatory Update on ABBV-951 (Foscarbidopa/Foslevodopa) New Drug Application | ABBV Stock News

AbbVie Provides Regulatory Update on ABBV-951 (Foscarbidopa/Foslevodopa) New Drug Application | ABBV Stock News
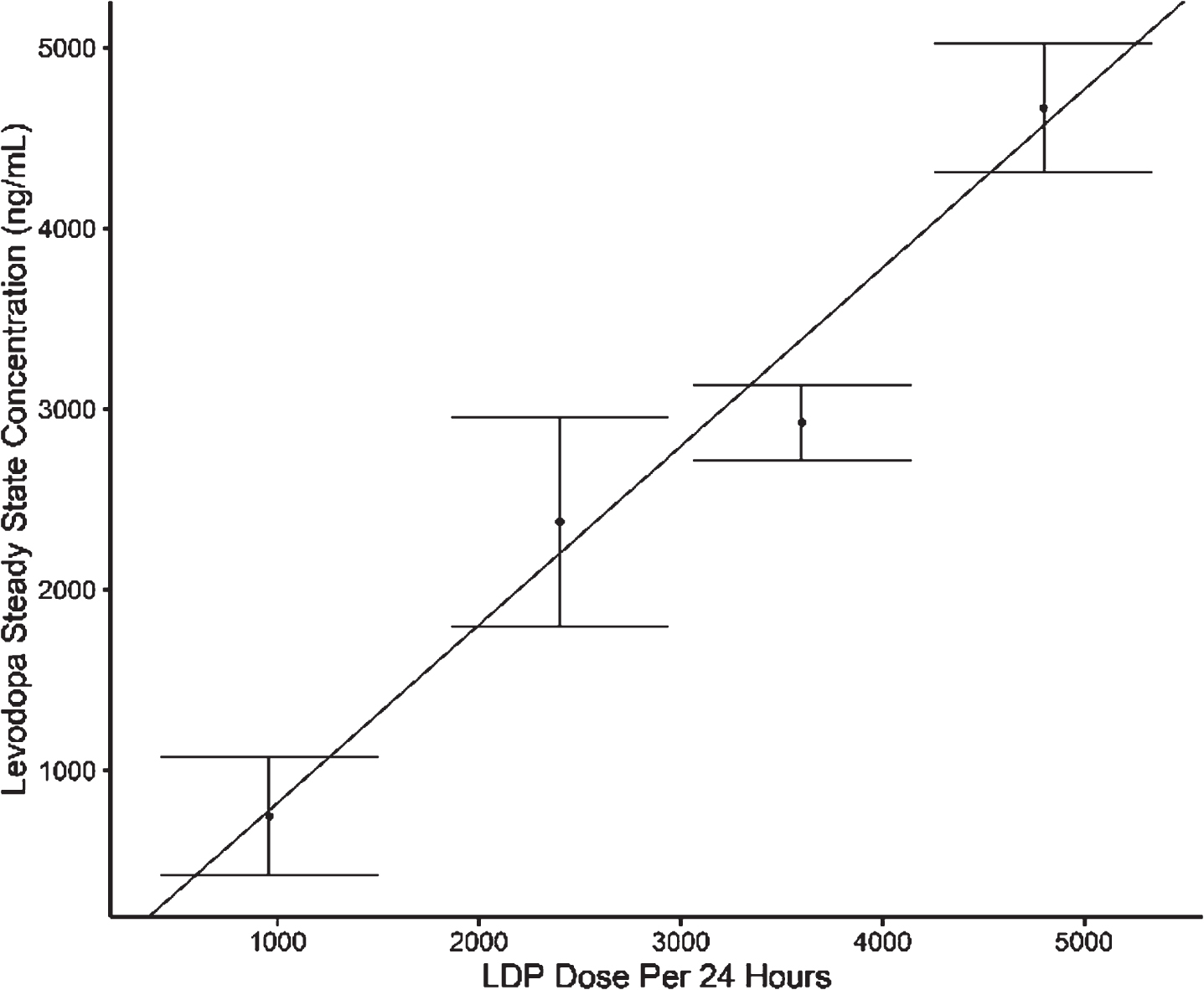
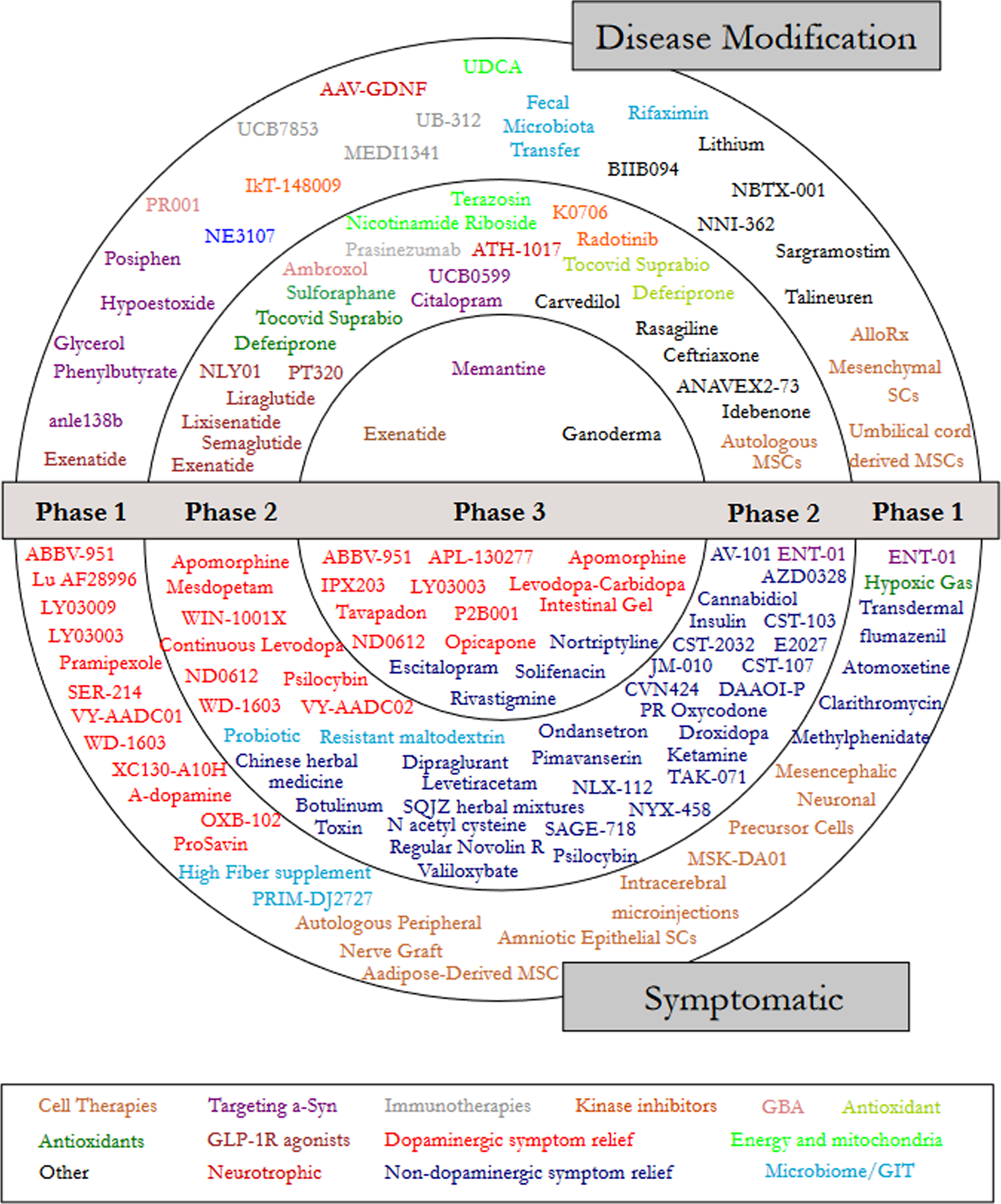
Foslevodopa/Foscarbidopa Is Well Tolerated and Maintains Stable Levodopa and Carbidopa Exposure Following Subcutaneous Infusion - IOS Press

Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson's disease: a randomised, double-blind, active-controlled, phase 3 trial - The Lancet Neurology

Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson's Disease - Rosebraugh - 2021 - Annals of Neurology - Wiley Online Library