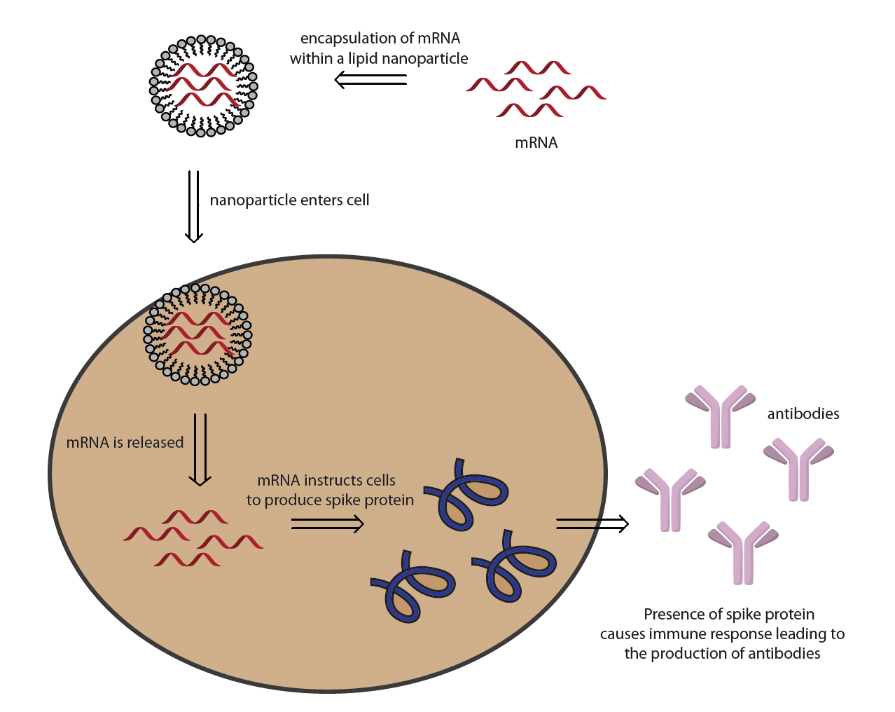
Ursula von der Leyen on X: "Our new 🇪🇺 contract 2021-2023 with @BioNTech_Group @Pfizer is now signed. It guarantees up to 1.8 billion doses for our fight against COVID and its variants.
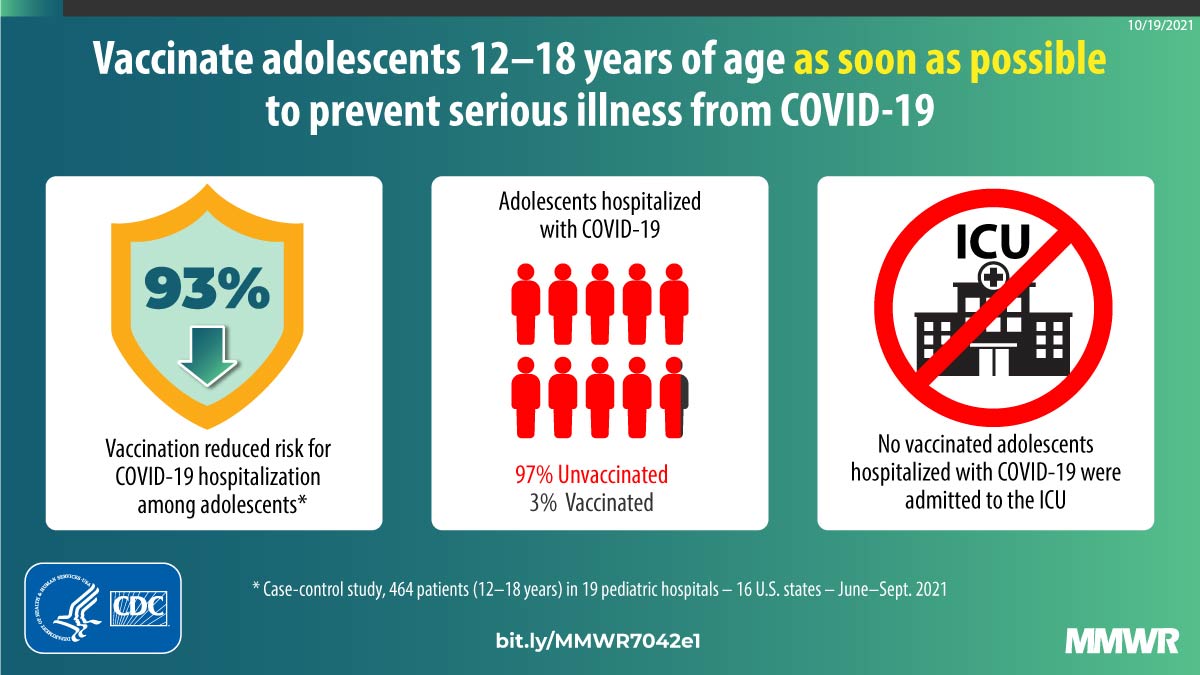
Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization Among Persons Aged 12–18 Years — United States, June–September 2021 | MMWR

Ministry of Health - Trinidad and Tobago - MOH Media Release: New WHO Approved COVID-19 Vaccine Combination. The Ministry of Health advises that, as at today, 30th July, 2021, the World Health

Chiricahua Community Health Centers, Inc. - PRESS RELEASE - FOR IMMEDIATE RELEASE September 30, 2021 At Chiricahua Community Health Centers, Inc. beginning on Friday October 1 2021, eligible persons (both patients and

FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose | Media call today at 10:45 a.m. ET