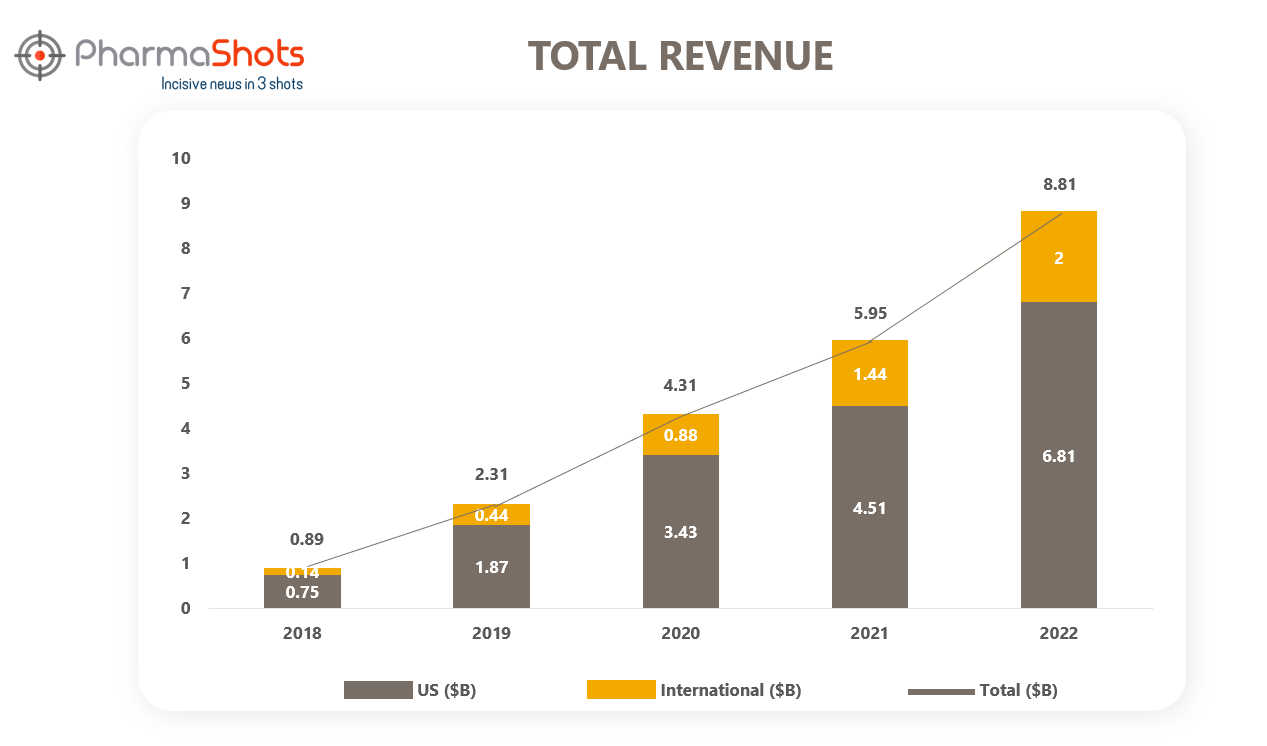
Regeneron Pharmaceuticals Inc.: Dupixent® (dupilumab) Late-breaking Phase 3 Data at the EADV 2022 Congress Showed Significant Improvements in Signs and Symptoms of Prurigo Nodularis - MoneyController (ID 826566)
Dupixent® (dupilumab) approved by European Commission as first and only targeted medicine for children as young as six months o

Sanofi SA: Dupixent® FDA approved as first and only treatment indicated for children aged 1 year and older with eosinophilic esophagitis (EoE) - Form 6-K - MoneyController (ID 1920828)
FDA Approves Dupixent® (dupilumab) for Moderate-to-severe Atopic Dermatitis in Adolescents | SnackSafely.com

APFED on Instagram: "Dupixent® FDA approved as first and only treatment indicated for children aged 1 year and older with eosinophilic esophagitis (EoE). #EoE https://www.sanofi.com/en/media-room/press-releases /2024/2024-01-25-19-30-00-2817342"
Dupixent® (dupilumab) application for treatment of chronic spontaneous urticaria (CSU) in adults and adolescents accepted for F

JAMA Dermatology Publishes Data Showing RINVOQ® (upadacitinib) Achieved Superiority Versus DUPIXENT® (dupilumab) for Primary and All Ranked Secondary Endpoints in Phase 3b Head-to-Head Study in Adults with Atopic Dermatitis | National Eczema

FDA approves Dupixent as first biologic medicine for children aged 6 months to 5 years with moderate-to-severe atopic dermatitis - PharmaLive

Dupixent® (dupilumab injection) now approved in Canada for the treatment of severe asthma in children aged six to 11 years with type 2 inflammation

FDA Approves Dupixent® (Dupilumab) as First Biologic Medicine for Children Aged 6 Months to 5 Years with Moderate-to-Severe Atopic Dermatitis | National Eczema Association