Bimekizumab for the treatment of moderate‐to‐severe plaque psoriasis: efficacy, safety, pharmacokinetics, pharmacodynamics and transcriptomics from a phase IIa, randomized, double‐blind multicentre study* - Oliver - 2022 - British Journal of Dermatology -

EMA Accepts Marketing Authorization Applications for UCB's Bimekizumab in Psoriatic Arthritis and Axial Spondyloarthritis - Practical Dermatology
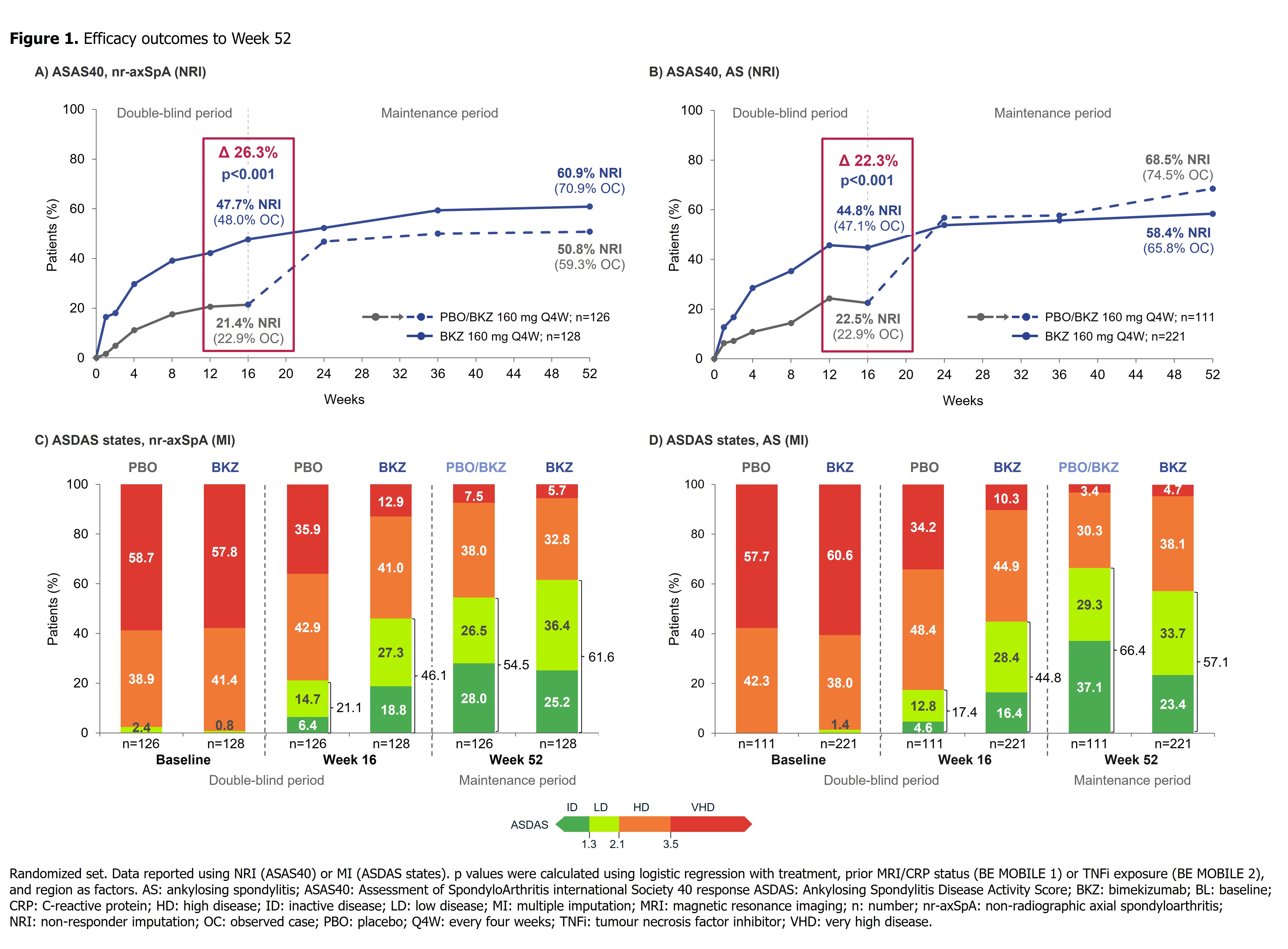
Bimekizumab Maintains Improvements in Efficacy Endpoints and Has a Consistent Safety Profile Through 52 Weeks in Patients with Non-Radiographic Axial Spondyloarthritis and Ankylosing Spondylitis: Results from Two Parallel Phase 3 Studies -

Chronic Plaque Psoriasis Market, Epidemiology, Clinical Trials And FDA Approvals By DelveInsight | Arcutis, Dermavant Sciences, UCB, BMS, Merck, Pfizer, Boehringer

Bimekizumab Leads to Rapid Improvements in AS Patients in Phase 3 Trials | Data Show AS Symptoms Reduced Within Week or 2 of Starting Bimekizumab | Ankylosing Spondylitis News
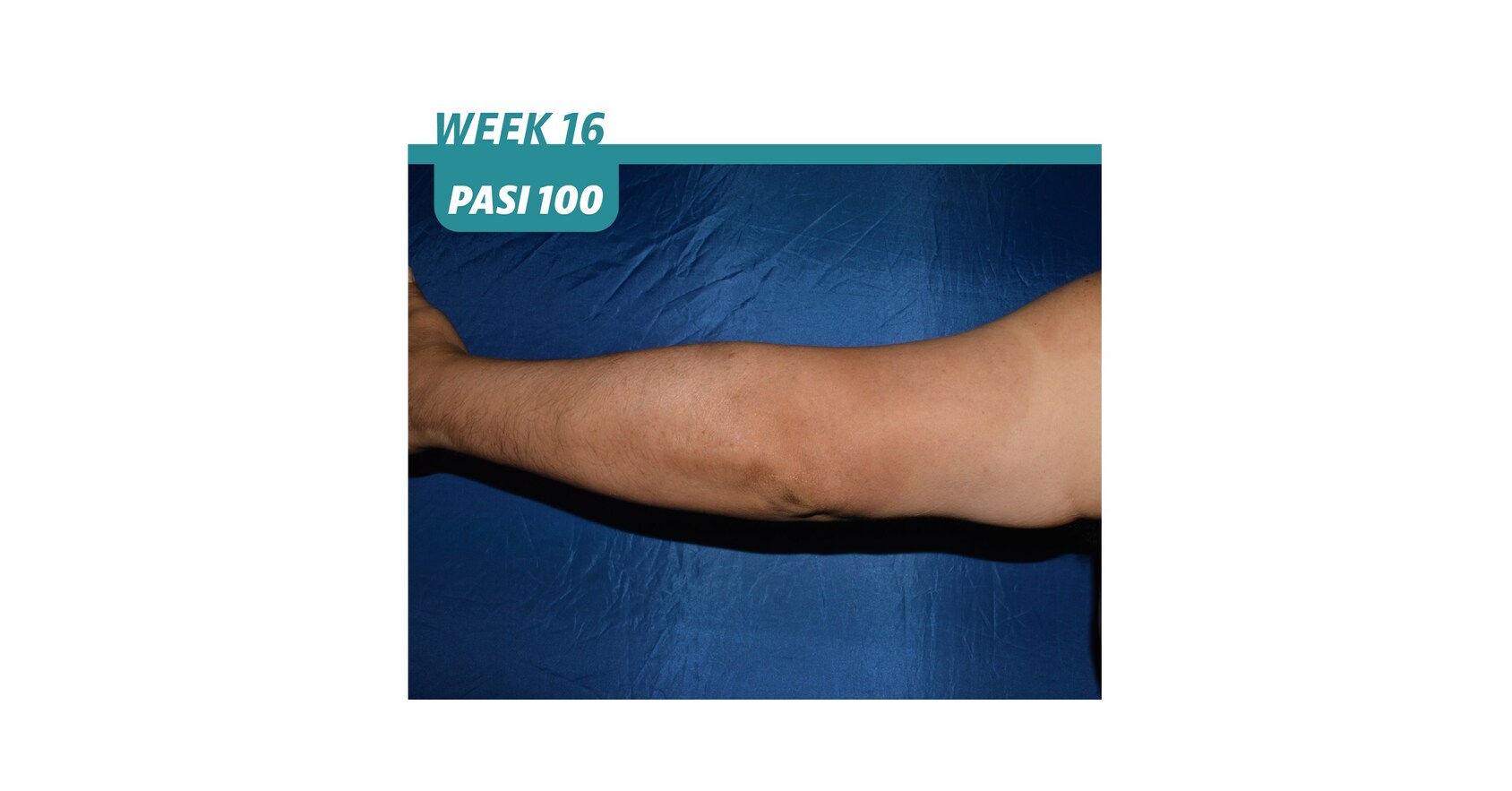
BIMZELX® Approved by the U.S. FDA for the Treatment of Adults with Moderate-to-Severe Plaque Psoriasis

Health Canada Approves BIMZELX® (bimekizumab) for the Treatment of Adults with Moderate to Severe Plaque Psoriasis
tranScrip congratulates UCB on receiving positive CHMP opinion recommending approval of BIMZELX[®*] (bimekizumab) in the EU - transcrip

UCB Receives the EMA's CHMP Positive Opinion Recommending Marketing Authorization of Zilucoplan for Adults with Generalized Myasthenia Gravis

Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial - The

BIMZELX® Approved by the U.S. FDA for the Treatment of Adults with Moderate-to-Severe Plaque Psoriasis

UCB's BIMZELX® Receives FDA Approval for Moderate to Severe Plaque Psoriasis - International Psoriasis Council